Approach fluidizes catalyst particles in electrolyte instead of gluing them to electrodes
By Alex Gerage // Originally published by Northwestern Engineering
Northwestern Engineering researchers have developed a more efficient and stable method to conduct electrocatalytic reactions.
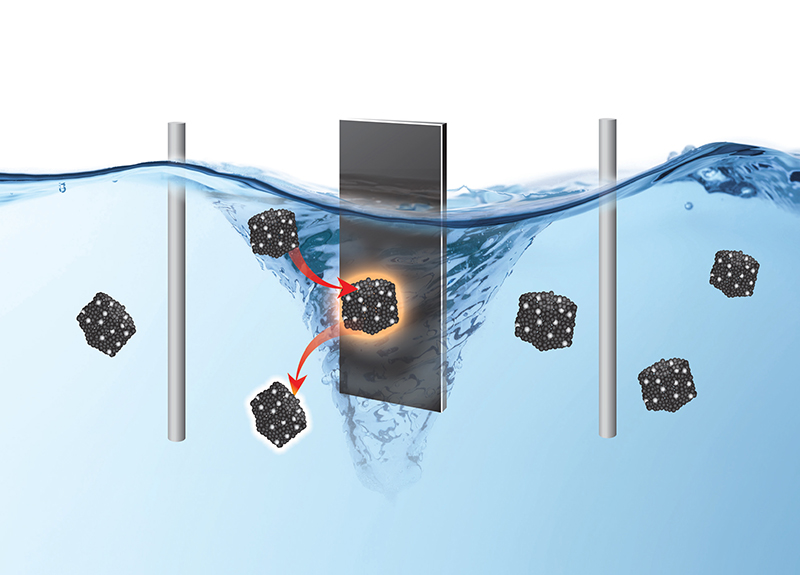
The technique, which fluidizes catalyst particles in electrolyte instead of gluing them to electrodes, avoids a rapid decline in reaction performance — a phenomenon researchers call fatigue. The approach could improve production processes for electrolysis and electrochemical energy conversion and storage.
“There has been extensive effort to find new high-performance catalysts that can also better withstand electrochemical reactions,” said Jiaxing Huang, professor of materials science and engineering at the McCormick School of Engineering, who led the research. “We developed a drastically different approach to make electrocatalysis less prone to decay — not by finding another new material, but by doing the reaction differently.”
The study, titled “Fluidized Electrocatalysis,” was published on February 10 in the journal CCS Chemistry and featured on the cover of the February issue.
In a typical electrocatalysis setting, once catalytic materials are glued onto the electrode, they are soaked in electrolyte and undergo a reaction spurred by a voltage. Since the voltage is continuously applied through the electrode, the materials experience continuous electrochemical stress. Over time, their catalytic performance can decay due to accumulated structural damage in the electrode as a whole, or on individual particles.
The team’s approach avoids the continuous stress by fluidizing the particles in the electrolyte. Now the particles work in rotation, experiencing electrochemical stress only momentarily when colliding with the electrode. Collectively, the output from the individual collision events merge into a continuous and stable electrochemical current.
“Fluidized electrocatalysis breaks the spatial and temporal continuum of electrochemical reactions, making the catalysts more efficient.” Huang said. “Fluidization also reduces the mass transport limit of the reactants to the catalyst, since the particles are swimming in the electrolyte.”
***
Prof. Huang is a researcher in the Center for Catalysis and Surface Science (CCSS), a strategic research center of the Institute for Sustainability and Energy at Northwestern (ISEN).