Article originally published by Michelle A. Harris in U.S. Department of Energy's Frontiers in Energy Research: Summer 2016. Harris is currently a postdoctoral researcher at Argonne-Northwestern Solar Energy Research Center (ANSER), an affiliate center of the Institute for Sustainability and Energy at Northwestern (ISEN).
A simple method for regenerating the cathode, a limiting factor in rechargeable batteries
From smart phones to electric cars, rechargeable batteries play a significant role in the world’s energy use. Unfortunately, such batteries do not last forever. The environmental and financial concerns of battery disposal have sparked research into prolonging their overall lifespan. One of the major limiting factors for rechargeable batteries is the degradation of the cathode. The Center for Mesoscale Transport Properties (m2M), an Energy Frontier Research Center, has demonstrated the most efficient cathode recycling in batteries.
When looking at a common battery, the cathode is the part labeled “+”. Over several cycles of recharging, the cathode loses capacity and holds less voltage. In lithium-based cathodes, this is typically caused by the loss of free lithium—leading to overcharging of the cathode. Overcharging the cathode contributes to structural defects and irreversible changes to the physical properties of the material.
Current methods for recycling the cathode are complicated processes that can involve hazardous acids and bases or completely destroying and remaking the cathode. Heat treatment is a less-destructive method for recycling the cathode but an effective method has not been found for common lithium-based cathodes because of their composition. Center director Esther Takeuchi and the research team at m2M developed a simple method for recycling a manganese oxide-based cathode using heat.
Their method for regenerating the manganese oxide-based cathode requires only two steps. First, they washed the cathode with dimethyl carbonate, a green chemistry solvent. Next, they heat the cathode at 300 ºC for two hours in open air. The presence of ambient air is a crucial detail. Why? Over several uses, the manganese oxide-based cathode loses oxygen and degrades structurally. Heat treatment in air restores the oxygen and restores the structure, or crystallinity.
The key to the manganese oxide cathode developed by m2M is its self-supporting structure where it can be separated from the battery, regenerated, and replaced (see figure). The battery used the manganese oxide cathode and a lithium metal anode. After discharging and recharging 50 times, the cathode was removed, heat treated, and replaced. They saw no degeneration of the battery after regenerating the cathode four times. This allowed the battery to last for more than 200 cycles.
After such positive results, the research team at m2M is hopeful that this method provides a conceptual approach that can be used more broadly. Takeuchi describes the research as “a new way of thinking about recycling batteries where an entire electrode can be regenerated and reused. We hope our results inspire further advances in this field.”
The m2M center’s method for recycling the cathode will lead to less battery waste and alleviate the cost of frequently replacing the battery. Their findings on the regeneration of the manganese oxide cathode shed light on regeneration for lithium and other metal cathodes and anodes as well. These results from m2M are revolutionary for the future of research and development of rechargeable batteries.
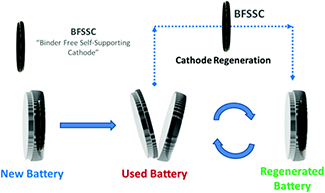 |
Diagram showing the recycling of the cathode in a coin-cell battery. Reproduced from Poyraz et al. (see below) with permission of The Royal Society of Chemistry. |
***
Acknowledgments:
This work was supported as part of the Center for Mesoscale Transport Properties, an Energy Frontier Research Center supported by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences. The transmission electron microscopy work was supported by DOE, Office of Science, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering. The X-ray photoelectron spectroscopy measurements used resources of the Center for Functional Nanomaterials, a DOE Office of Science user facility, at Brookhaven National Lab.
More Information:
Poyraz AS, J Huang, S Cheng, DC Bock, L Wu, Y Zhu, AC Marschilok, KJ Takeuchi, and ES Takeuchi. 2016. “Effective Recycling of Manganese Oxide Cathodes for Lithium Based Batteries.” Green Chemistry 18:3414-3421. DOI: 10.1039/C6GC00438E
Photo credit: James Almond



